Evaluating Enhanced Efficiency N Fertilizers under Different Moisture Condition
Research was funded by the Tennessee Department of Agriculture (Land and Water Stewardship Programs)
Nutifafa Adotey, Assistant Professor and Soil and Nutrient Specialist, Samuel Okai, Graduate Students, Xinhua (Frank) Yin, Professor & Cropping System Scientist, Daniel Yoder, Professor, Lori Duncan, Assistant Professor and Row Crop Sustainability Specialist, Debasish Saha, Assistant Professor and Soil Scientist
Introduction
Nitrogen can easily be lost in the soil system through several loss pathways such as ammonia volatilization, denitrification, surface leaching, and runoff. Thus, for most supplemental N fertilizer are required for optimal crop productivity. Surface-applied, unincorporated, urea-based N fertilizers are commonly used N fertilizers in crop production mainly because of its limited logistical constraints. The major drawback for these fertilizers is their susceptibility to ammonia volatilization if it is unincorporated. Ammonia volatilization starts with the hydrolysis of urea by the urease enzyme to form ammonium bicarbonate. The resulting alkaline microclimate favors the conversion of ammonium to ammonia gas. One of the tools available to control ammonia loss particularly in no-till production systems that are vulnerable to ammonia loss is enhanced efficiency nitrogen fertilizers (EENF). These fertilizers slow the urea hydrolysis process before appreciable amounts of N can be lost as ammonia. In Tennessee, split application of N is recommended where growers apply more than 120 lb N/A during the growing season. Most of the recommended N is applied between V4-V6 growth stage and delaying N fertilizer beyond this recommendation application window can reduced grain yield. Heavy rainfall during this optimal widow can create muddy or flooded conditions. Applicating urea-based fertilizers is inefficient in flooded soil conditions partly because of higher ammonia volatilization. Numerous studies have been published on the effectiveness EENF in reducing ammonia loss under dry soil conditions. However, there is limited information on the efficacy of EENF forms of urea-based fertilizers to reduce ammonia loss in muddy or flooded soil conditions for growers that have options for aerial N application. The objective of this study was to evaluate the inhibitory effect of urea-based EENF under different soil moisture conditions.
Experiment
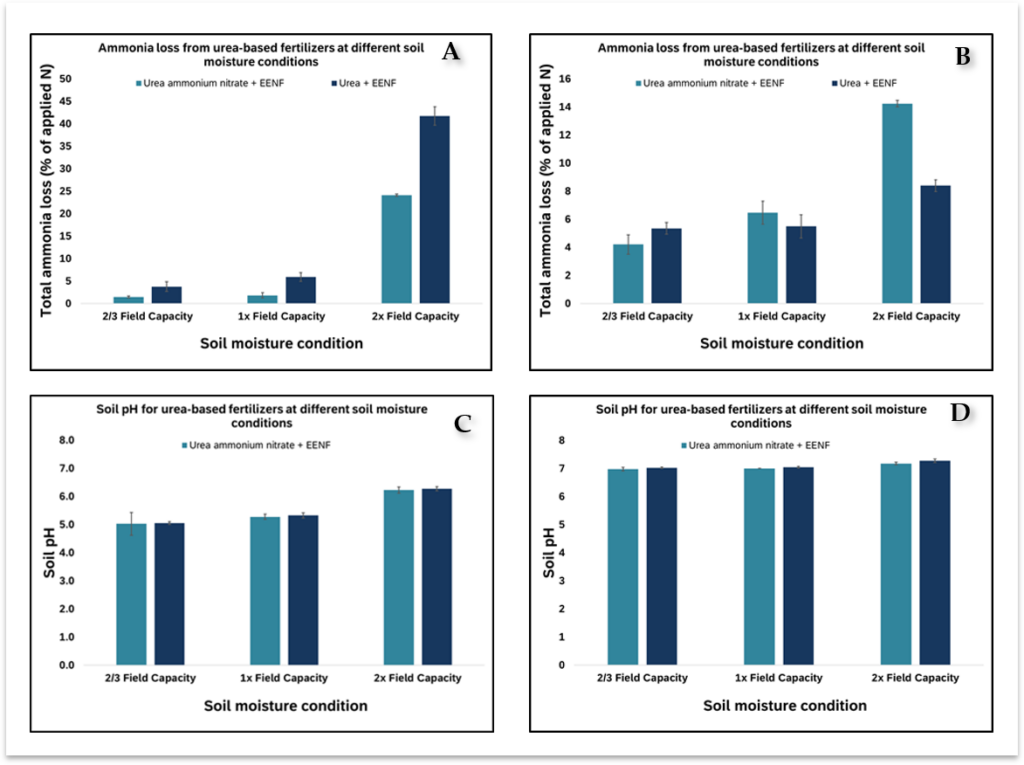
The soils used in this experiment were classified as a Loring silt loam (Fine-silty, mixed, active, thermic Oxyaquic Fragiudalfs) and Grenada silt loam (Fine-silty, mixed, active, thermic Oxyaquic Fraglossudalfs) using the USDA soil classification system (Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture, 2024). Preplant soil samples were collected at the depth of 0 – 15 cm and analyzed for soil pH and Mehlich 3 extractable nutrients (Southern Cooperative Series, 2014), which is shown in Table 1. Trial was set up as a randomized complete block design with four replicates to examine the efficacy of enhanced efficiency nitrogen fertilizer (EENF) forms of urea and UAN applied at three different soil moisture conditions for a total of 24 experimental units (n = 2 x 3 x 4). The EENF used in this experiment was ANVOLTM (Koch Agronomic Service, Wichita, KS). The moisture conditions evaluated include (i) 2/3 x (ii) 1 x, and (iii) 2 x the field capacity of the soil. Ammonia volatilization was measured using a rapid and precise in-lab controlled environment system (Figure 1), similar to systems described by Woodward et al. (2011). The temperature was maintained at 28 °C, air flow rate through the system was set at 1 L/m, and the air was near 100 % saturation.

Results
In Loring silt loam (pH 5.8), ammonia loss from urea was significantly higher than UAN at all the moisture conditions (Figure A). However, in the Grenada silt loam (pH 7) the ammonia loss from UAN was similar to urea at all the moisture content except at the 2x FC (Figure B). Ammonia loss from UAN was significantly higher than urea at the 2x FC. This suggests that the performance of EENF (urea and UAN) on ammonia volatilization under flooded conditions depends on the initial pH of the soil as well as the fertilizer source. Soil pH is instrumental in the equilibrium between ammonium and ammonia with alkaline soil pH conditions favoring the conversion of ammonium to ammonia and vice versa. Urea ammonium nitrate contains only 50% urea in addition to 25% nitrate and 25% ammonium. So, when UAN is applied on soils with pH greater than 7, the onset of ammonia loss begins right away because of the availability of ammonium. However, in low pH (<6) soils with their higher concentrations of H+ ions favor the conversion of ammonia to ammonium ion, which minimizes ammonia loss. Unlike UAN, other soil properties such as soil buffering capacity may have greater influence than the initial soil pH. When urea-based fertilizer is applied onto the soil with adequate moisture and favorable temperature, the compound breaks down into ammonium bicarbonate through a process called urea hydrolysis. The hydroxide ion increases soil pH around the granules and creates a perfect condition for the conversion of ammonium into ammonia. In poorly buffered soil, rapid ammonia volatilization occurs and vice versa from well buffered soil.
Generally, ammonia loss increased with soil moisture content, however the loss was more pronounced at the 2x field capacity, where the N fertilizer was applied into muddy or standing water. In low pH soil (5.8), there was no difference between the 2/3 and 1x FC at corresponding N fertilizer, which were significantly lower than 2x. In the higher pH soil (7.0), ammonia loss increased significantly from each moisture level for UAN. There was no difference between the 2/3 and 1x FC, which were lower than 2x FC. The higher losses can be attributed to an increase in soil pH as results of flooding at the 2x FC (Figure B). When soils with pH less than 7 are flooded, the H+ are consumed during the reduction process so the soil pH increases and then levels off or may fluctuate (Figure C). As soil pH increases, hydroxide ions favor the conversion of ammonium to ammonia gas as observed in. In high pH soil, significant differences are not observed among soil pH.
Conclusion
Overall, this research demonstrates that the EENF forms of urea and UAN evaluated in this study are not ideal when applied into muddy or flooded conditions. It is important that EENF forms of urea and UAN are surface broadcast onto dry ground and not onto muddy ground or standing water, because such conditions increase the rate of ammonia loss.





